
FDA Approval For MDMA Is Coming Next Year: Here Are The 3 Companies Positioned To Profit Most
The Multidisciplinary Association for Psychedelic Studies (MAPS) has been at the forefront of the medical psychedelic industry forward for years, and their clinical investigation of MDMA for the treatment of PTSD is poised to be the first true psychedelic compound to gain FDA approval. On the heels of MAPS’ recently completed Phase 3 study of “molly” in PTSD patients, a billionaire baseball team owner has committed $5M to the non-profit association to establish a financial incentive program to ensure that this groundbreaking new therapy is accessible to everyone once approved. This is not the first time that big wig investors have written large checks to fund psychedelic research, both in the form of grants to research institutions and investments in biotech startups. The stigma of psychedelics has officially been stripped, and this burgeoning field is going to re-define mental health treatment and offer many attractive investment opportunities in a highly fragmented industry. Approval of MDMA is just the start.
Introduction
From caves to the clinic
Psychedelic use has a rich and diverse history, spanning ancient ceremonial practices to the discovery of LSD. Throughout human civilization, various cultures have employed natural substances like psilocybin mushrooms, ayahuasca, and peyote for spiritual, healing, and transformative purposes. These substances were believed to provide access to higher states of consciousness and facilitate communication with the divine. However, it was not until the mid-20th century that the modern era of psychedelics began with the accidental discovery of lysergic acid diethylamide (LSD) by Albert Hofmann. LSD quickly gained attention for its profound and mind-altering effects, leading to extensive research and exploration of its therapeutic potential, artistic inspiration, and cultural impact. While psychedelic use has encountered periods of stigma and prohibition, recent years have witnessed a resurgence of interest, with scientific studies highlighting their therapeutic benefits and a renewed understanding of their potential for personal growth and spiritual exploration.
Learn more about the history of psychedelics
Classification
Psychedelic medicines can be broadly classified into several categories based on their chemical composition and functional effects. These classifications help in understanding the unique properties and mechanisms of action of each psychedelic compound. Here are some of the main categories:
- Serotonergic psychedelics: This category includes substances that primarily interact with the serotonin system in the brain. Examples include LSD (lysergic acid diethylamide), psilocybin (found in magic mushrooms), and DMT (N,N-Dimethyltryptamine), the active component in ayahuasca. Serotonergic psychedelics generally bind to serotonin receptors, particularly the 5-HT2A subtype, leading to altered perception, enhanced introspection, and mystical experiences.
- Dissociatives: These substances induce a state of dissociation from the environment and the self. Common dissociatives include ketamine, PCP (phencyclidine), and DXM (dextromethorphan). They primarily work by antagonizing NMDA receptors, leading to a disruption of sensory perception and producing an anesthetic, out-of-body experience.
- Entactogens: Also known as empathogens, elicit feelings of emotional openness, empathy, and connectedness. MDMA (3,4-Methylenedioxymethamphetamine), commonly known as ecstasy or molly, is the most well-known entactogen. It primarily acts on serotonin, dopamine, and norepinephrine systems, enhancing emotional well-being and promoting social bonding.
- Deliriants: Deliriants are characterized by inducing a state of delirium and confusion, often accompanied by hallucinations. Substances like diphenhydramine (Benadryl) and Datura contain deliriant properties. They primarily antagonize acetylcholine receptors, leading to altered cognition, vivid hallucinations, and memory impairment.
- Other psychedelics: This category encompasses a variety of substances that produce psychedelic effects but may not fit neatly into the aforementioned categories. Examples include mescaline (found in peyote and San Pedro cacti), ibogaine (derived from the iboga plant), and 5-MeO-DMT (a more potent variant of DMT). Each of these compounds has unique chemical structures and mechanisms of action, resulting in diverse subjective experiences.
Learn more about the science of medical psychedelics
It's important to note that these classifications are not rigid, and some substances can exhibit overlapping effects or characteristics from multiple categories. Additionally, research on psychedelics is an ongoing process, and our understanding of their mechanisms and classifications continues to evolve.
3,4-Methylenedioxymethamphetamine
More than a party drug
MDMA (3,4-Methylenedioxymethamphetamine), commonly known as ecstasy or molly, has been the subject of extensive research and clinical trials for its potential therapeutic applications. MDMA is classified as an entactogen, known for its empathogenic and euphoric effects. In clinical settings, MDMA-assisted psychotherapy has shown promising results in the treatment of post-traumatic stress disorder (PTSD). Clinical trials have demonstrated that MDMA, when used in conjunction with therapy, can help patients address and process traumatic memories and improve their emotional well-being. These studies have shown significant reductions in PTSD symptoms, with long-lasting benefits observed even after the MDMA-assisted sessions. MDMA-assisted therapy has also shown potential in the treatment of anxiety-related disorders and other mental health conditions.
Clinical trials
MDMA's unique properties, including its ability to enhance empathy and emotional openness, can facilitate a therapeutic process. The drug promotes a sense of trust, safety, and connectedness, allowing individuals to explore and process traumatic memories and emotions that contribute to their psychological distress. Clinical trials have shown promising results. For example, the Multidisciplinary Association for Psychedelic Studies (MAPS) conducted Phase 3 trials that involved participants with treatment-resistant PTSD. These trials found that MDMA-assisted psychotherapy resulted in significant reductions in PTSD symptoms. MAPS anticipates full FDA approval of MDMA to treat PTSD in the first half of 2024.
In addition to PTSD, there is also growing interest in exploring the use of MDMA-assisted therapy for other mental health conditions such as anxiety-related disorders, depression, autism spectrum disorder, and end-of-life distress in individuals with life-threatening illnesses. While research is still ongoing, the preliminary preclinical findings suggest that MDMA-assisted therapy has the potential to provide significant benefits in these areas as well.
MindMed (NASDAQ: MNMD), (NEO: MMED) is advancing a proprietary MDMA compound, MM-402, for the treatment of autism spectrum disorder (ASD) in a preclinical model of the disease. This R-enantiomer version of MDMA possesses less stimulant activity than the native MDMA, allowing for at-home dosing with low risk of side effects (such as heart palpitations, shortness of breath, and increased blood pressure). Preliminary preclinical data suggests that MM-402 could enhance social functioning and become a first-in-class therapy for the core symptoms of ASD.
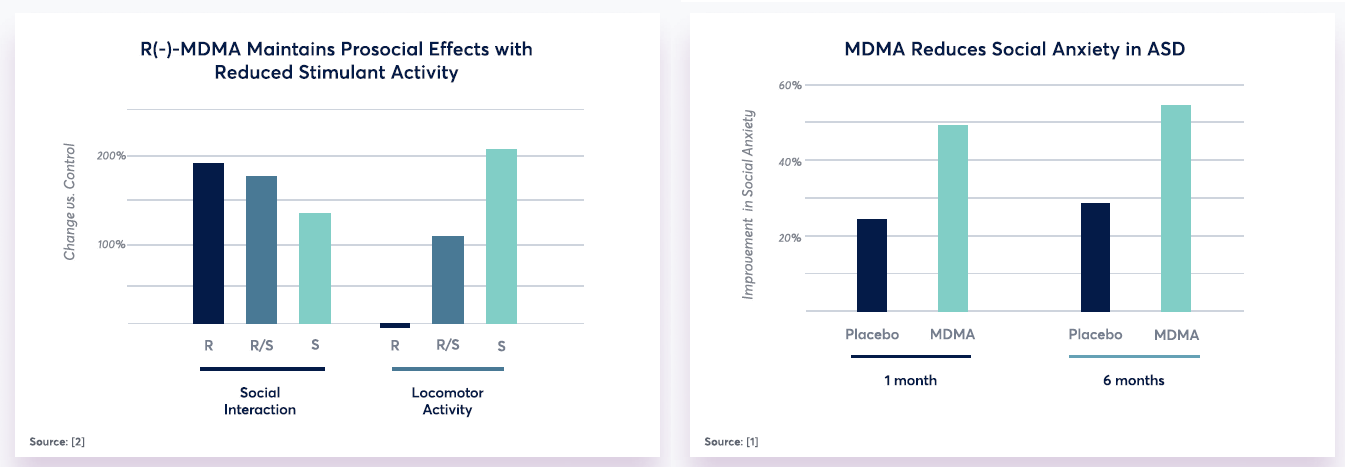
In addition to MindMed’s preclinical research program for MM-402, their collaborators University Hospital Basel (“UHB”) in Switzerland are enrolling participants in a Phase 1 investigator-initiated trial of R-MDMA, S-MDMA and R/S-MDMA in healthy volunteers. This Phase 1 trial is a randomized, placebo-controlled, double-blind, 5-period crossover study which will enroll 24 healthy participants who will each receive doses of MM-402 (125 and 250 mg), S-MDMA (125 mg), MDMA (125 mg), and placebo. Acute subjective effects in this study are being assessed using the Visual Analog Scales (“VAS”) and the 5 Dimensions of Altered States of Consciousness (“5D-ASC”) along with measurement of autonomic, endocrine and mood effects, among others. Additional information about this trial is on clinicaltrials.gov (identifier: NCT05277636).
Learn more about the most advanced clinical studies of medical psychedelics
MNMD shares continue to frustrate both bulls and bears as it consolidates within a symmetrical triangle pattern. Resistance up near $3.80 with strong support at ~$3.00 - this is a chart to watch for an eventual upside resolution later in the year as we get closer to MM-120 Phase 2 topline readouts.

It's important to emphasize that the therapeutic use of MDMA is conducted in a clinical and controlled environment, with careful attention to safety, appropriate dosing, and integration of the therapy sessions. This approach is distinct from recreational or non-medical use of MDMA, which can carry its own risks. Overall, the research and clinical trials on MDMA-assisted psychotherapy are shedding light on the potential of MDMA as a tool for healing and psychological transformation, offering new possibilities for individuals struggling with treatment-resistant mental health conditions.
MDMA manufacturing
The introduction of MDMA in the clinic is imminent, and MAPS anticipates MDMA becoming available for psychedelic assisted therapies (PAT) in the first half of 2024. There will be clear demand for GMP-grade MDMA and PAT-trained therapists to administer the therapy.
PharmAla Biotech (CSE:MDMA) is positioned to capture the commercial MDMA manufacturing market by leveraging its pre-existing infrastructure and relationships formed as the largest supplier MDMA to clinical trial sites in AU. Its materials have already been approved by Human Research Ethics Committees (HRECs) in several major universities within Australia, allowing it to be used in human clinical studies.
MDMA-assisted psychotherapy is expected to cost USD $10,000-$15,000 per 3-month course, with the drug representing $1,500 of the total cost. While the therapy is likely to be covered by insurance, the MDMA drug product will remain an out-of-pocket expense for the foreseeable future. PharmAla expects the North American, Caribbean, and European markets to add to their revenues in exponential fashion over the next three years (see figure below). The company estimates USD$350,000 in clinical trial supply revenues for 2023, and the European and Caribbean markets to add an additional USD $1-2M to their top line. PharmAla is also the exclusive global broker for MindSet’s GMP psilocybin drug product, offering potential revenues of up to CAD $250K in 2023.

While PharmAla’s main focus appears to be on commercial manufacturing of MDMA (and its analogs) for research, clinical studies, and patient use, the company is also working on a proprietary MDMA compound. Similar to MindMed’s R-MDMA variant (MM-402), the company’s lead drug candidate, ALA-002, has demonstrated significantly improved cardio- and neuro-toxicity profiles compared to MDMA in animal model of autism. These improvements, in addition to a significant reduction in hyperthermia, solve the three major adverse events associated with MDMA. PharmAla expects to move directly into a Phase 2 as an Investigational New Drug (IND) opening study (i.e., first-in-human clinical trial) based on the improved safety profile of ALA-002.
MDMA shares continues to be the standout performer in the Canadian medical psychedelics space in 2023. The May/June correction appears to have concluded, and the uptrend has resumed. Let's see if MDMA can make a new all-time high above $.54 in the 2H of 2023.

MDMA in the clinic
Numinus Wellness (TSX: NUMI) is the best positioned network of psychedelic-assisted therapists to leverage the approval of MDMA to rapidly expand their USA and CA clinics. On June 10, 2022, Numinus acquired Novamind, a network of integrative mental health clinics and contract research organization specializing in psychedelic medicine. This purchase added eight new wellness clinics to Numinus's existing five centers, as well as two additional research sites for the conduct of clinical trials. Not only did this acquisition expand Numinus's presence into the USA, but they also attained CRO capabilities via Cedar Clinical Research. The transaction was immediately accretive to Numinus's revenue and gross profit, growing the former by 464% (compared to the previous quarter) for a total of $4.2M in 4Q22. Quarterly revenues continued to swell to $5.7M in 1Q23, and gross profit margin grew to 41.9% from 31.5% in 4Q22 (company quarterly report).

Numinus recently announced a partnership with the Multidisciplinary Association for Psychedelic Studies (MAPS) to conduct a clinical study of their MDMA-assisted therapy education program in Canada. This "Experiential Opportunity" trial will be made available to trained psychedelic-assisted therapy practitioners to assess the benefit of Numinus's proprietary MDMA education program. Titled A Phase 1, Open-Label, Single-Arm Study to Evaluate MDMA Experiential Training in Healthy Volunteers and Expand Knowledge and Qualifications of Therapists Planning to Conduct MDMA-Assisted Therapy (NUMT1), will be conducted at Numinus' clinics in Vancouver, and may expand to other clinics in their network in the future. The clinical trial protocol will allow each participant to both receive MDMA-assisted therapy under the supervision of a trained therapist as well as observe another participant receiving the therapy.
"If the trial protocol is approved, Numinus would be best positioned to provide training with our experiential opportunities. Practitioners who complete our MDMA-assisted therapy education program, or have already completed previous MDMA-assisted therapy programs and are qualified to enroll in our Practical Applications course, would be able to apply to participate in the experiential clinical trial – enabling them to observe, deliver, and receive the therapeutic protocol as part of their training experience."
~ Payton Nyquvest, Founder and CEO
The formalization of psychedelic-assisted therapy (PAT) protocols is a critical step in advancing medical psychedelics into therapeutic settings. Numinus's study is a first-of-its-kind, and will catalyze the adoption of MDMA as a first-line treatment for PTSD. The ultimate win for the industry is the codification of PAT protocols in the DSM-V.
"Our 37-year journey to medicalize the therapeutic use of psychedelics has never been closer to a reality, and we're thrilled to have Numinus as a partner in this work. Experiential training is an important element of a practitioner's training in many therapeutic modalities, and through this carefully controlled clinical trial, Numinus is supporting our shared goal of providing training to practitioners who may one day deliver psychedelic-assisted therapy to individuals who need it."
~ Rick Doblin, Founder and President of MAPS
NUMI shares display clear signs of accumulation, and the ~50% YTD gain is impressive considering how out of favor the medical psychedelics sector has been throughout the first half of 2023.

Conclusion
The prospect of FDA approval for MDMA and the transformative potential of psychedelic-assisted therapy underscore the shifting landscape of mental health treatment. As MAPS leads the way with Phase 3 trials and expects FDA approval, the acceptance of MDMA as a therapeutic tool holds immense promise. Companies like PharmAla Biotech, Numinus Wellness, and MindMed are strategically positioned to capitalize on this evolving industry, whether through commercial manufacturing or the expansion of psychedelic-assisted therapy clinics. With the stigma surrounding psychedelics dissipating, robust research, clinical trials, and investments are reshaping the field and offering new possibilities for patients and investors alike.
As the medical community delves deeper into the therapeutic properties of psychedelics, the potential impact on mental health treatment becomes increasingly evident. FDA approval for MDMA and other psychedelic compounds have the potential to revolutionize mental health care, providing hope for individuals with treatment-resistant conditions. With the trajectory of the medical psychedelic industry pointing towards growth, it is crucial to approach this emerging field with caution and conduct comprehensive research to navigate the opportunities and challenges that lie ahead. Ultimately, the convergence of scientific exploration, regulatory milestones, and investment potential signifies an exciting time for the field of psychedelic medicine.
Disclosure
MedicalGold.ca has not received compensation from any company mentioned in this article. We thank our site sponsors for helping to support our ability to create high quality content like the article you are reading.
Author owns shares of MNMD at the time of publishing and may choose to buy or sell at any time without notice.
DISCLAIMER:
The work included in this article is based on current events, technical charts, company news releases, and the author’s opinions. It may contain errors, and you shouldn’t make any investment decision based solely on what you read here. This publication contains forward-looking statements, including but not limited to comments regarding predictions and projections. Forward-looking statements address future events and conditions and therefore involve inherent risks and uncertainties. Actual results may differ materially from those currently anticipated in such statements. This publication is provided for informational and entertainment purposes only and is not a recommendation to buy or sell any security. Always thoroughly do your own due diligence and talk to a licensed investment adviser prior to making any investment decisions. Junior resource and biotechnology companies can easily lose 100% of their value so read company profiles on www.SEDARplus.ca for important risk disclosures. It’s your money and your responsibility.
