
Top 5 Psychedelic Late-Stage Clinical Trials To Keep An Eye On in 2023 - (1H23 Update)
Update
8/10/2023
- MAPS has reported results from long-term observational follow-up on MDMA-assisted therapy for PTSD (press release). While the data has still not been published, these interim results "show that participants in this study demonstrated a durable response at least six months, and in some cases a year or more, after their final MDMA-assisted therapy session during the Phase 3 study.”
The abysmal psychedelic stock market belies the monumental advancements in psychedelic medicine that have been made within the last two years. It is absolutely clear that psychedelic biotech valuations are completely divorced from fundamentals. Multiple research papers have confirmed then validity of psychedelic-inspired drugs as treatments for mental illnesses on a hard, quantitative level [1, 2]. There are almost 500 ongoing clinical studies of psychedelic substances and psychedelic-assisted therapies (often in combination), and the industry leaders (discussed below) have catapulted multiple drug assets (LSD, ketamine, DMT, psilocybin, and MDMA) into Phase 2 (26 ongoing) and Phase 3 (2 ongoing) clinical trials. Recent regulatory changes in favor of “compassionate use” have already allowed psychedelics to be administered to suffering patients. The industry is fully legitimized and on the cusp of a critical inflection point: regulatory approval of the first psychedelic therapy.
Without further ado, here are the top psychedelic clinical trials (both ongoing and recently completed) to keep an eye on in 2023:
Top 5 Clinical Trials
1. MAPS' Phase 3 study of MDMA for PTSD
(in collaboration with Numinus)
Study title
A Multi-Site Phase 3 Study of MDMA-Assisted Therapy for PTSD (MAPP2)
Status
Completed on 11/17/2022, top-line results not yet available - expected to be published in Q1 2023.
Interim report published on 4/5/2023. Still waiting on publication of data.
Study design
- Multi-site, double blind, randomized, placebo-controlled.
- 12 week treatment period preceded by 3 preparatory sessions with a therapist.
- A total of 3 experimental sessions were conducted for each participant.
- 104 participants were randomized to two groups who received either: (1) an initial dose of 80 to 120mg MDMA hydrochloride followed by a supplemental half-dose of 40 or 60mg approximately 1.5hr into the extended therapy session, or (2) a placebo plus extended therapy sessions.
- Each experimental session was followed by 3 "integrative" sessions.
- PTSD symptoms measured by multiple scales, including the Clinician-Administered PTSD Scale (CAPS-5) as the primary endpoint, and Sheehan Disability Scale of functional impairment as the secondary endpoint.
Preliminary results
- No serious adverse events observed in the MDMA group.
- Results met study pre-specified primary and key secondary endpoints
- New Drug Application (NDA) submission expected in Q3 2023.
- These results confirm the findings of MAPS' first Phase 3 study [3] (data below), MAPP1, in which 88% of participants responded to the treatment and robust attenuation of CAPS-5 was observed with MDMA (P < 0.0001, d = 0.91) and significant decrease in SDS total score (P = 0.0116, d = 0.43). The mean change in CAPS-5 scores in participants completing treatment was −24.4 (s.d. 11.6) in the MDMA group and −13.9 (s.d. 11.5) in the placebo group. MDMA did not induce adverse events of abuse potential, suicidality or QT prolongation.
- MAPS' two pivotal Phase 3 studies, MAPP1 and MAPP2, are the only completed Phase 3 trials of a psychedelic-assisted therapy in the entire sector. New Phase 2 trials are being designed to evaluate MDMA-assisted therapies for other mental illnesses, such as substance use disorder and eating disorders. These additional Phase 2 trials will determine if MDMA-assisted therapies may be effective for other conditions or with other treatment modalities commonly used to address PTSD.
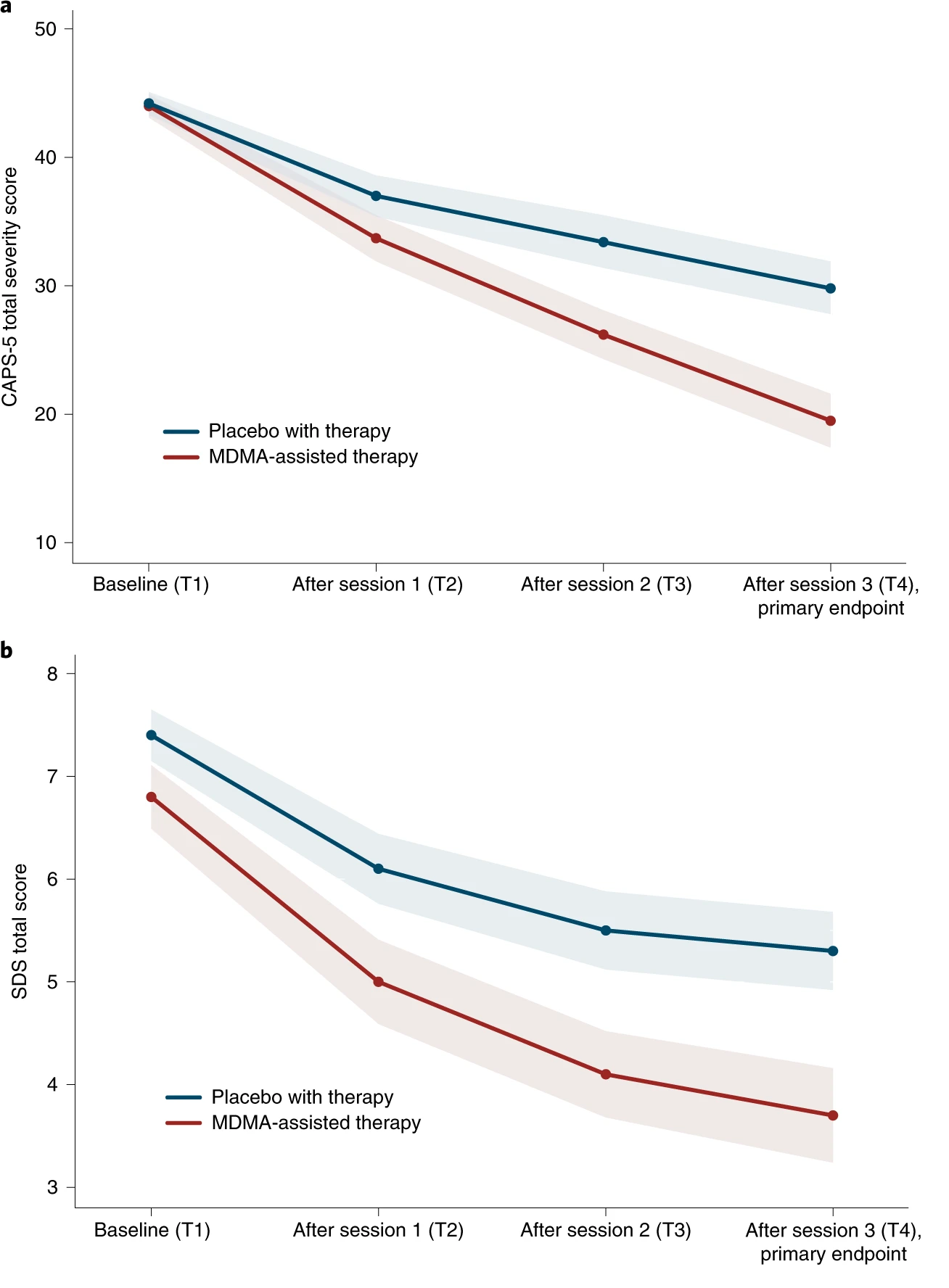
Significance
We should expect a New Drug Application from MAPS in 2H23, and if approved this will be the first medical psychedelic commercialized since Sprovato (nasal ketamine) in March 2019, and the first ever psychedelic-assisted therapy for PTSD (an ever growing problem especially among US military veterans). In June 2022, Numinus jumped to the front of the industry with their acquisition of Novamind, which gave them a network of 12 wellness clinics supported by psychedelic-assisted therapy (PAT)-trained clinicians capable of administering MDMA therapy sessions. A successful MDMA approval from MAPS would give Numinus full access to administer MDMA in their treatment clinics; a first of its kind. Once MDMA is validated as a safe and efficacious treatment for PTSD, MAPS will be positioned to quickly pursue other related disease indications.
"The Phase 3 confirmatory results support the development of MDMA-assisted therapy as a potentially new breakthrough therapy to treat individuals with PTSD—a patient population that is often left to suffer for years. Now with two positive Phase 3 trials complete, we can add this important data to the new drug application which we expect to submit in the third quarter of this year."
~ Amy Emerson, chief executive officer, MAPS PBC
Read MedicalGold's full coverage of Numinus here.
2. Compass Pathways' Phase 3 study of psilocybin for TRD
Study title
Status
Recruiting patients, estimated completion date October 2024.
Study design
- Multi-center, double blind, randomized, placebo-controlled.
- Study will last up to 16 weeks, including a three- to ten-week Screening Period and six-week follow-up from investigational psilocybin product (ICOMP360) administration.
- 378 participants randomized in a 2:1 ratio to receive COMP360 at a dose of 25mg or placebo.
- Primary endpoint: Montgomery-Åsberg Depression Rating Scale (MADRS) (a 10-item clinician rated scale measuring depression severity) at Week 6.
- Secondary endpoint: Sheehan Disability Scale (SDS) is a brief, five-item self report inventory that assesses functional impairment in work/school, social life, and family life.
Preliminary data
- Phase 3 data is not available yet.
- Compass's Phase 2b results for COMP360 as a treatment for treatment-resistant depression (TRD) were published to the New England Journal of Medicine on November 3, 2022 [4], and were used to inform the design of the ongoing Phase 3 study.
- The Phase 2b results demonstrated a single 25mg dose of COMP360 psilocybin treatment caused 30% of TRD patients to enter remission at Week 3, and a sustained response (24%) was seen through Week 12 in 2x the number of patients that received 25mg vs 1mg (demonstrating a dose-related effect; see data below).
- However, adverse events occurred in 179 of 233 participants (77%) and included headache, nausea, and dizziness. The vast majority (>90%) of these treatment emergent adverse events (TEAEs) were mild/moderate, but suicidal ideation or behavior or self-injury occurred in all dose groups (total of 12 patients; note: there was no placebo group). Still, Compass claims that COMP360 was generally well-tolerated because suicidal ideation and intentional self-injury are extremely common in treatment-resistant depression studies [Link].
- After the publication of the Phase 2b results, Compass's stock plummeted by 24%... perhaps a "buy the rumor and sell the news" phenomenon... or maybe there is good reason to be skeptical of the significance of their data. First, let's give credit where credit is due. The 25mg of COMP360 produced a fast and durable response, eliciting anti-depressive effects within 2 days and sustaining a substantial (although perhaps not clinically meaningful response) at 12 weeks post-dose. The difference between depressive symptoms at Week 3 demonstrated the greatest disparity between the 25mg and 1mg groups (-6.6 change from baseline in MADRS score, both clinically meaningful [6] and statistically significant, p<0.001). However, less than half of patients in the 25mg group responded at Week 3, and less than a quarter sustained the response at Week 12, suggesting that COMP360 is effective in a dose-dependent manner but it is no panacea for TRD (which is completely understandable... TRD patients have already failed to response to two SSRIs. These patients are difficult to treat and are riddled with comorbidities). A bit more concerning is the high percentage of adverse events that appear to correlate to dose level, which we believe is what panicked investors when they read "suicidal behavior" and "self-injury in the press release. However, only 1 of the 12 patients had a TESAE within 24 hours of dosing. The other 11 experienced their TESAEs up to 62 days after the dose, suggesting that the emergence of serious depressive symptoms is not an acute, drug-related effect, and may be related to other variables.

“Our additional analyses underline the robustness of our findings that a single high dose of COMP360 psilocybin, given in conjunction with psychological support, led to a rapid and sustained response for many patients. This phase IIb study was designed to determine the optimal COMP360 dose for our phase III programme, evaluating safety and efficacy at the primary endpoint at week 3. Additionally, we observed consistent improvement in measures of anxiety, positive and negative affect, quality of life, daily functioning, cognition, and self-reported depression. We believe this could make a tremendous difference to patients suffering with treatment-resistant depression, who have few options available to them. Remember, a quarter of the 25mg group maintained response, as measured by the MADRS, at 12 weeks after a single administration with no other antidepressant medication. This finding in itself is unprecedented.”
~ Guy Goodwin, Chief Medical Officer, COMPASS Pathways
Significance
While Compass is fighting an uphill battle to prove that their psilocybin-based investigational drug, COMP360, can produce a clinically meaningful and sustained response while mitigating treatment-related adverse events, they are the best positioned company to gain approval for the first psilocybin therapy for patients suffering from treatment-resistant depression (TRD). TRD is especially pernicious because it remains impervious to existing anti-depressants, and patients are at increased risk for comorbid anxiety disorder, higher suicide risk, as well as deleterious metabolic changes [5]. A steep hill to climb indeed, but Compass appears confident that their Phase 2b data is encouraging enough to launch a 2-trial pivotal Phase 3 program [Link]. Once approved for TRD, Compass may decide to pursue additional clinical trials for major depressive disorder (MDD), which will likely show positive results since MDD is a less severe form of depression than TRD. Alternatively, COMP360 may be prescribed off-label for MDD at physcians' discretion.
3. MindMed's Phase 2b study of LSD for anxiety
Study title
Status
First patient dosed on August 25, 2022.
Study design
- Multi-center, double blind, randomized, placebo-controlled.
- Study will last up to 12 weeks.
- 200 participants randomized across 5 study arms: (1) placebo, (2) 25ug MM-120 (LSD), (3) 50ug MM-120, (4) 100ug MM-120, or (5) 200ug of MM-120.
- Primary endpoint: to determine the dose-response signal and assess the dose-response relationship of 4 doses of MM-120 (25, 50, 100 or 200 μg free base equivalent) as measured by the change in HAM-A Total Score from Baseline to Week 4.
- Secondary endpoints: to determine the dose-response signal and assess the dose-response relationship of 4 doses of MM-120 (25, 50, 100 or 200 μg free base equivalent) as measured by the change in HAM-A Total Score from Baseline to Week 8 and Week 12 (end of study); Improved Quality of Life as measured by EuroQol 5 Dimension; Assess the incidence of Adverse Events (AEs) and Serious Adverse Events (AEs)5 Level (EQ-5D-5L).
Preliminary data
- Preliminary Phase 2b data is not available yet.
- MindMed's pilot Phase 2a study was published on September 2, 2022 [7], and investigated the effects of 200ug MM-120 (LSD) on anxiety at 16 weeks using the Spielberger State-Trait Anxiety Inventory–Global score in 42 patients.
- In this pilot Phase 2a study, investigators observed significant reductions in anxiety scores up to 16 weeks after treatment [7].

Significance
MindMed stands to commercialize the first LSD pharmaceutical since Delysid lost its patent in the 1960's. This will legitimize the use of Indolealkylamines, which include psilocybin and DMT in addition to LSD, and are based on the core serotonin structure.
Read MedicalGold's full coverage of MindMed.
4. Small Pharma's Phase 2a study of DMT for MDD
Study title
Status
Completed on 12/22/2022, and top-line data released on 1/25/2023 (summary of the results below).
Updated open-label results 3/7/2023 - Further analysis of the Phase IIa dataset is ongoing and full trial results will be submitted for publication in a peer-reviewed journal.
Study design
- Multi-center, double blind, randomized, placebo-controlled.
- 34 participants randomized across two study arms: (1) a single dose of SPL026 (21.5mg) delivered via IV infusion (n=17) or (2) placebo.
- The two-staged Phase 2a study included a placebo-controlled phase, where the primary endpoint was to assess the efficacy of a single dose of SPL026 with supportive therapy (N=17) versus placebo with therapy (N=17) at two-weeks post-dose.
- All study participants were subsequently enrolled into an open-label phase of the study where they received a single dose of SPL026 with supportive therapy, and were followed-up for a further 12-weeks in study.
- This open-label trial design enabled the assessment of durability of antidepressant effect, as well as the comparative efficacy and safety of a one versus two dose regimen of SPL026.
- Efficacy was assessed using the Montgomery-Asberg Depression Rating scale (“MADRS”) to measure any potential change in patients’ depression from baseline. MADRS was assessed by independent raters who were not present at dosing and were blinded to the overall treatment.
Preliminary data
Updated 3/7/2023
- The Phase 2a study met the primary endpoint demonstrating a statistically significant and clinically relevant [6] reduction in depressive symptoms two-weeks following a dose of SPL026 with supportive therapy, compared to placebo, demonstrating a -7.4 point (clinically meaningful) difference in MADRS (p=0.02).
- Analysis of key secondary endpoints demonstrated a rapid onset of antidepressant effect one-week post-dose, with a statistically significant difference in MADRS score between the active and placebo groups of -10.8 (p=0.002).
- Across the 12-week open-label phase, patients who received at least one active dose of SPL026 with supportive therapy reported a durable improvement in depression symptoms (57% remission rate at 12-weeks following a single dose of SPL026).
- No apparent difference in antidepressant effect was observed between a single and two dose regimen of SPL026.
- The total mean reduction in MADRS from baseline after a single dose of SPL026 was –15.4 at 12-weeks.
- SPL026 was well tolerated by all patients receiving an active dose.
- No drug-related serious adverse events reported, including no reported suicidal ideation or behavior. All adverse events related to treatment were considered mild or moderate.
- Analysis of patient-reported depression scores corroborate the MADRS assessments conducted by independent clinical raters. Improvements in depression scores from baseline were observed across all study timepoints in patients receiving at least a single dose of SPL026, as measured by the Beck Depression Inventory (“BDI”), including a statistically significant improvement in depression symptoms compared to placebo at two-weeks post-dose (p=0.002). The efficacy outcomes on the BDI were consistent with MADRS, providing additional support for the rapid and sustained therapeutic profile of SPL026 for the treatment of MDD.
- Measures assessing patients’ anxiety and wellbeing, areas which are often negatively impacted by depression, were also analyzed across the study. Following both one and two doses of IV SPL026 with supportive therapy, patients demonstrated a rapid and sustained improvement in anxiety symptoms as measured by the State-Trait Anxiety Inventory-Trait (“STAI-T”) scale. A statistically significant improvement in anxiety symptoms was observed compared to placebo at two-weeks post-dose (p=0.03). At 12-weeks following the open-label dose, a -14.2 mean change from baseline (“CFB”) was demonstrated in the patient group receiving the single dose regimen.
- Further, a rapid and sustained improvement in wellbeing was observed following at least a single treatment of IV SPL026 with supportive therapy, as measured by the Warwick-Edinburgh Mental Wellbeing Scale (“WEMWBS”). The results at two-weeks following the blinded dose of SPL026 or placebo showed a 10.1 mean CFB in the SPL026 group compared to 0.9 in the placebo group.
Final results
Updated open label results 4/3/2023
- Patients followed out to 6 months (open-label), enabling further assessment of the durability of the antidepressant effect. A total of 25 patients from both the single and two-dose regimen completed the 6 month follow up.
- 14 patients achieved remission within the 3 month in-study period.
- 9 of these remitters sustained remission at 6 months.
- Overall, 10/25 patients (40%) met the criteria for remission at 6 months.

Significance
This is the first placebo-controlled efficacy study completed to date that investigates a short-duration psychedelic for depression and demonstrates both a rapid (effects seen at 1 week) and durable response (effects endured up to 12 weeks). The change in MADRS score for depression is astonishingly high, more than double that of COMP360 (psilocybin) in Compass Pathway's DMT for treatment-resistant depression (TRD) trial (not to draw a direct head-to-head comparison between the two studies, as TRD is a completely different beast compared to major depressive disorder and much less tractable).
We are pleased that a significant number of patients benefited from the treatment in our trial. SPL026 with supportive therapy was shown to have a significant antidepressant effect that was rapid and durable, with a remission rate of 57% at three months following a single dose of SPL026. It was encouraging to see that SPL026 demonstrated a favourable safety and tolerability profile in MDD patients in this study, consistent with our Phase I study. The results are clinically meaningful and enable us to progress into an international multi-site Phase IIb study where we seek to further explore the efficacy and safety profile of SPL026 in a larger MDD patient population.”
~ George Tziras, Chief Executive Officer of Small Pharma
5. Tryp's Phase 2 study of psilocybin for BED
Study title
Status
Ongoing, interim results of first 5 patients announced on 1/5/2023 (see data below).
Study design
- Open label study (i.e., both clinician and patient know that the patient is receiving the investigational drug).
- 10 participants receive a single 25mg oral dose of TRP-8802 (psilocybin) is administered in a monitored setting following 6-8 hours of preparatory psychotherapy.
- Subjects will be in the study for 12 weeks following the dose of TRP-8802 (i.e., until Week 14).
- Primary endpoint is to assess the safety of a single dose of TRP 8802 in participants with BED during the TRP 8802 dosing session, and through 12 weeks following dosing.
- Secondary endpoints: (1) Evaluate the feasibility of inducing the psychedelic state with TRP 8802 in a BED population, (2) Determine the preliminary clinical activity and the effects of TRP 8802 in conjunction with psychotherapy on the frequency of binge eating episodes in a BED population through 4 weeks following dosing, and (3) Determine the preliminary clinical activity and the effects of TRP 8802 in conjunction with psychotherapy on weight-related indicators in a BED population through 4 weeks following dosing.
- These secondary endpoints will measure the (1) Magnitude and duration of TRP 8802-induced dissociative effects in participants with BED using the MEQ30 and MRS, (2) Frequency of binge eating episodes as measured by a modified Eating Questionnaire, and (3) Waist Circumference and BMI of patients, respectively.
Preliminary data
- The first patient in the STOP trial exhibited reduced overall anxiety, reduced anxiety around food, reduced compulsion to overeat and improved self-image and confidence.
- Analysis of the additional four patients has reinforced the initial clinical observations.
- Across all patients, daily binge eating episodes were reduced by an average of 80.4% from baseline during the four-week post-dosing measurement period, with all patients reporting a daily reduction in binge eating episodes of at least 60% from baseline.
- 4 of 5 patients reported at least a 75% reduction in daily binge eating episodes from baseline during the four-week post-dosing measurement period.
- The number of daily instances of patients feeling that they had lost control over their eating were reduced by an average of 81.6% during the four-week post-dosing measurement period, with 4 of 5 patients reporting a reduction of greater than 70%.
- The current results demonstrated a significant reduction in the frequency of binge eating behavior for each patient as measured in multiple assessments of efficacy which were discussed with the FDA as acceptable endpoints in advance of this study.
- In addition, analysis of the Hospital Anxiety and Depression Scale (HADS) anxiety and depression scores demonstrated improving trends related to patients' levels of anxiety and depression. The observed behavioral improvements are consistent with those described in other clinical studies examining the clinical benefit of psilocybin as a therapeutic intervention in compulsion-related disorders.
- There were no drug-related adverse events reported by these patients during the four-week period following dosing of TRP-8802.
Significance
These results affirm that binge eating disorder (BED) is a viable target for psychedelic-assisted psychotherapy using a psilocybin/psilocin analog. Tryp's proprietary IV formulation of psilocin, TRP-8803, is positioned to be the first psychedelic pharmaceutical that overcomes the limitations of oral psilocybin by reducing the time to onset and controlling the duration of the experience.
"The magnitude and consistency of the trends observed in this interim analysis are incredibly encouraging. Furthermore, these preliminary results provide us with the confidence that BED is a viable target for future studies with psychedelic-assisted psychotherapy utilizing TRP-8803, our proprietary IV formulation of psilocin that alleviates numerous shortcomings of oral psilocybin including: significantly reducing the time to onset of the psychedelic state, controlling the depth and duration of the psychedelic experience, and reducing the overall duration of the intervention to a commercially feasible timeframe. Our strategy is to perform small exploratory studies using TRP-8802 for unique indications including BED, fibromyalgia and irritable bowel syndrome, all in partnership with leading academic institutions. Once a positive clinical signal is identified in studies using TRP-8802, we intend to perform subsequent studies with TRP-8803."
~ Jim Gilligan, Ph.D., Tryp's CEO
Conclusion
These late-stage clinical trials present unequivocal evidence that psychedelic-inspired pharmaceuticals have potent efficacy against a wide range of mental ailments, and will be the next revolution in psychiatric treatment. We are witnessing an arms race of sorts, wherein multiple companies are developing similar technologies against a relatively small set of overlapping disease indications. This is not an unprecedented phenomenon (just look at the cancer immunotherapy space and PD-1 inhibitors), and it is truly a great sign for the entire industry as more attention and capital flows into the sector seeking a competitive edge. The first approval of a DMT, psilocybin, or LSD-based compound will herald a new age of enlightenment for mental health practitioners and their suffering patients, opening the floodgates of R&D and igniting the smoldering capital markets.
While these industry leaders pave the way, there are many smaller psychedelic biotechs that will benefit from the tailwinds created by their big brothers.
Stay tuned for MedicalGold's "Top 5 Early-Stage Clinical Trials To Watch In 2023".
References
[1] Daws, R.E., Timmermann, C., Giribaldi, B. et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med28, 844–851 (2022). https://doi.org/10.1038/s41591-022-01744-z [Link]
[2] Davis AK, Barrett FS, May DG, et al. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 2021;78(5):481–489. doi:10.1001/jamapsychiatry.2020.3285 [Link]
[3] Mitchell, J.M., Bogenschutz, M., Lilienstein, A. et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med27, 1025–1033 (2021). https://doi.org/10.1038/s41591-021-01336-3 [Link]
[4] Goodwin, G. M., Aaronson, S. T., Alvarez, O., Arden, P. C., Baker, A., Bennett, J. C., Bird, C., Blom, R. E., Brennan, C., Brusch, D., Burke, L., Campbell-Coker, K., Carhart-Harris, R., Cattell, J., Daniel, A., DeBattista, C., Dunlop, B. W., Eisen, K., Feifel, D., … Malievskaia, E. (2022). Single-dose psilocybin for a treatment-resistant episode of Major Depression. New England Journal of Medicine, 387(18), 1637–1648. https://doi.org/10.1056/nejmoa2206443 [Link]
[5] Jaffe, D.H., Rive, B. & Denee, T.R. The humanistic and economic burden of treatment-resistant depression in Europe: a cross-sectional study. BMC Psychiatry19, 247 (2019). https://doi.org/10.1186/s12888-019-2222-4 [Link]
[6] Turkoz, I., Alphs, L., Singh, J., Jamieson, C., Daly, E., Shawi, M., Sheehan, J. J., Trivedi, M. H., & Rush, A. J. (2021). Clinically meaningful changes on depressive symptom measures and patient‐reported outcomes in patients with treatment‐resistant depression. Acta Psychiatrica Scandinavica, 143(3), 253–263. https://doi.org/10.1111/acps.13260 [Link]
[7] Holze F, Gasser P, Müller F, Dolder PC, Liechti ME; (n.d.). Lysergic acid diethylamide-assisted therapy in patients with anxiety with and without a life-threatening illness: A randomized, double-blind, placebo-controlled phase II study. Biological psychiatry. Retrieved January 26, 2023, from https://pubmed.ncbi.nlm.nih.gov/36266118/ [Link]
DISCLAIMER:
The work included in this article is based on current events, technical charts, company news releases, and the author’s opinions. It may contain errors, and you shouldn’t make any investment decision based solely on what you read here. This publication contains forward-looking statements, including but not limited to comments regarding predictions and projections. Forward-looking statements address future events and conditions and therefore involve inherent risks and uncertainties. Actual results may differ materially from those currently anticipated in such statements. This publication is provided for informational and entertainment purposes only and is not a recommendation to buy or sell any security. Always thoroughly do your own due diligence and talk to a licensed investment adviser prior to making any investment decisions. Junior resource and biotechnology companies can easily lose 100% of their value so read company profiles on www.SEDARplus.ca for important risk disclosures. It’s your money and your responsibility.
