
Cybin Inc: Transforming "Psychedelics to Therapeutics™" With Psilocybin and DMT

The time is nigh...
We have been watching Cybin Inc. closely for the last year, waiting for them to advance out of the preclinical stage and begin human clinical trials for their psychedelic-inspired drug candidates. With the recent acquisition of a DMT Phase 1 clinical trial program and the dosing of their first patients in their major depressive disorder study, Cybin has now emerged as one of the most formidable contenders in the medical psychedelic space. MedicalGold has decided to initiate coverage at this valuation inflection point for the company, and we will continue to follow them as they leverage their enormous compound library to transform "Psychedelics to Therapeutics™".
Cybin Inc. (NEO:CYBN) (NYSE:CYBN) is a leading ethical biopharmaceutical company, working with a network of world-class partners and internationally recognized scientists, on a mission to create safe and effective therapeutics for patients to address a multitude of mental health issues. Headquartered in Canada and founded in 2019, Cybin is operational in Canada, the United States, the United Kingdom, and Ireland. The Company is focused on progressing Psychedelics to Therapeutics by engineering proprietary drug discovery platforms, innovative drug delivery systems, novel formulation approaches, and treatment regimens for mental health disorders. Cybin currently has three active drug development programs (CYB003 for major depressive disorder and alcohol abuse, CYB004 for anxiety disorders, and CYB005 for neuroinflammation) supported by 189 preclinical studies. The company owns over 50 novel, proprietary psychedelic compounds.
Table of Contents:
An International Health Crisis
Depression & anxiety
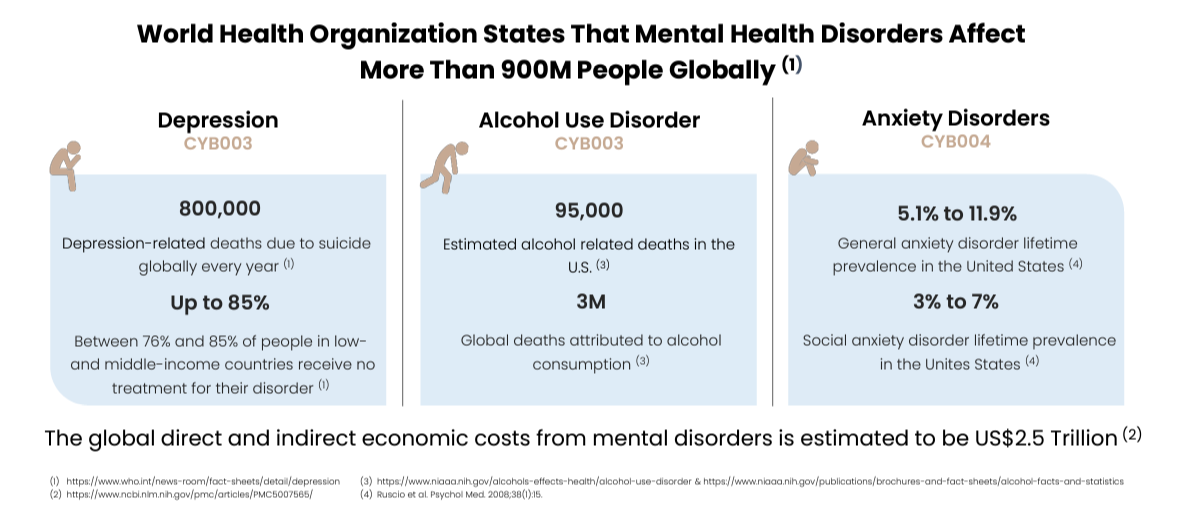
Mental health remains a rapacious killer, despite the stigma slowly peeling away and awareness campaigns encouraging people to seek help. Major Depressive Disorder (MDD) is estimated to affect 5% of adults worldwide, and is responsible for 800,000 suicides every year. Disturbingly, suicide is the 4th leading cause of death in adolescents and young adults ages 15-29 yrs [1]. Statistics aside, we all know someone that has suffered (or have first-hand knowledge) from bouts of mood fluctuations that surpass the "normal" variegations that are a part of normal life. Depression is caused by a mix of psychological, environmental, and sociological factors. The complexity of the disease was made shockingly apparent during the COVID-19 crisis, during which many people who could not fathom mental illness were suddenly thrust into its throes.
This uptick in mental health issues was studied by Cigna (the health insurance company) who found that anti-anxiety and anti-insomnia prescriptions increased 21% between February 16th and March 15th of 2020, coinciding with the official declaration of the pandemic [2]. A study by the American Psychiatric Association concluded that 62% of people felt more anxious in 2021 than in 2020 [3]. Even before the COVID pandemic, health officials were concerned with the rising rates of benzodiazepine (Xanax, Klonopin) prescriptions due to the staggering increase in fatal overdoses from 1999 to 2018 (1,135 v. 10,724 deaths) [4]. Recent data published by the US Census Bureau and National Center for Health Statistics shows that poll respondents showing symptoms of anxiety or depression have risen 4-fold compared to pre-pandemic figures.
Anti-anxiety medications (i.e., anxiolytics; e.g., benzodiazepines, or "benzos") provide fantastic relief in the short term, but are highly addictive with chronic use. It is truly alarming how freely psychiatrists will prescribe these medications, but not surprising given their immediate results (who doesn't want a patient to report instant improvement?). Benzos are intended for emergency use, and are not a suitable treatment for for abiding distress or to mask the symptoms of a more insidious personality disorder (e.g., bipolar disorder). Here's one physician's take on the matter:
"Some benzodiazepine prescribing is straightforward, for example, as a brief intervention for acute distress or as-needed use for a phobic anxiety (e.g., airplanes) or transient insomnia. Some prescribing situations are, however, to be avoided if possible, like prescribing to manage persisting distress resulting from a personality disorder or for patients with known current or past substance use disorders. But prescribing is never carefree..."
~ Jerrold F. Rosenbaum, MD and psychiatrist at Mass General Hospital [Link]
At least anxiolytics are effective. That's more than can be said for the antidepressant medications that have been doled out for the last 50 years. The efficacy of the most commonly prescribed antidepressants are hotly debated and the data is inconclusive at best. The exact causes of depression are not well understood, but researchers have been able to attack the disease using pharmacological agents with various mechanisms of action. Three major classes of pharmaceuticals exist: tricyclics (TCAs), selective serotonin re-uptake inhibitors (SSRIs), and selective serotonin noradrenaline re-uptake inhibitors (SNRIs). A study published in The Lancet examined the remission rates of 3.6M patients undergoing mono-treatment with one of these common antidepressants (e.g., Amitriptyline, Bupropin, Citalopram, Doxepin, Escitalopram, Fluoxetine, Sertraline, Trazadone, and a handful more), and determined that the variance between patient subgroups (such as examining patients within a certain age range, or grouping them by gender) confounded any meaningful measurement in improvement [5]. A meta-analysis published in 2022 found that health-related quality of life of patients treated with antidepressants did not meaningfully improve over a 12-year timespan [6].
"Published clinical trials have stated that the utilization of psychopharmacotherapy may improve the clinical outcomes of patients. However, the evidence around the beneficial overall effect for these medications in patients with depression disorder is controversial. Multiple meta-analyses were conducted to assess the change in outcomes for patients treated for MDD including different antidepressant medications. These studies concluded that most of the improvement on symptoms (about 80%) came from the placebo effect for these medications [17–19]. They found that the difference between the placebo and treatment groups was very minimal in the meta-analyses that included data from published studies [17–19] and when data from unpublished studies were combined with data from published studies the difference became statistically insignificant, or even clinically undetectable [17, 19]. Moreover, when psychotherapy was evaluated against antidepressants, it was found to have a comparable efficacy to prescribed antidepressants [17]."
~ Antidepressants and health-related quality of life (HRQoL) for patients with depression: Analysis of the medical expenditure panel survey from the United States [Link]
The modest benefits that patients may receive from antidepressants must also be weighed against the inevitable side-effects. Besides the classic dry mouth, headache, and dizziness, antidepressants can also cause sleep disturbances, impotence, and suicidal ideation. Yes, that's right. Taking antidepressants can actually put you at more risk for harm (talk about a binary outcome...). Clearly, there is a need for a new class of pharmaceuticals to treat major depressive disorder and anxiety disorders with greater efficacy, more reliability, and fewer side effects.
Alcohol Use Disorder (AUD)
Alcohol addiction remains a global threat with 3M global deaths related to alcohol consumption, 95,000 of which occurred in the USA [7]. There's a line between casual consumption of alcohol and full blown addiction, but it can become blurry when encroached upon. Alcohol Use Disorder (AUD) is diagnosed subjectively, whereby the physician tries to discern how much power alcohol has over the patient's decision making. Get ready to feel judged. The National Institute on Alcohol Abuse and Alcoholism defines binge drinking as consuming 4 or more alcoholic drinks in a given day [8]. We have all been binge drinkers at one time or another, but most of us have not had a problem putting the bottle down. Like depression, the causes of AUD are both Nature and Nurture; a combination of genetic predisposition to addiction and circumstance (e.g., high stress job, a floundering relationship, a global pandemic, etc.) determine a person's likelihood to experience AUD, and their response to treatment.
To date, there are three FDA-approved treatments for AUD: naltrexone (oral and long-acting injectable), acamprosate, and disulfiram. These three drugs have different mechanisms of action, but all are intended to increase the chances that alcohol addicted patients are able to maintain their sobriety. Unfortunately (but not surprisingly), all three are also associated with a litany of side-effects on their warning labels, including (but not limited to) liver failure (disulfiram), resistance to opioids (naltrexone), and renal failure (disulfiram, acamprosate) [9]. The effects of acamprosate and naltrexone are modest, and even though disulfiram has been used for years, the clinical evidence is inconsistent [10]. Most physicians will agree that the clinical data is not compelling enough to justify these risks, yet there are no other options and their hands are tied. The need for a new treatment modality for AUD is pressing.
The Solution: Medical Psychedelics
The sacred shroom
Psychedelics have been regarded as sacred plants since the dawn of civilization, with the earliest known consumption of plants containing psychedelic agents occurring in 5000 BCE in Southeastern Algeria. The plants which contain these psychedelic agents include mushrooms, ayahuasca, cacti, morning glory vines, and even some grasses. Psychedelic ceremonies have shaped many religions and are still used throughout the world to invoke spiritual experiences (ayahuasca ceremonies are particularly en vogue these days, with Westerners flocking to the Amazon in Peru to receive the medicine under the direction of shamans. Seven thousand years' of use is quite a testament to the power of plant-derived psychedelics.
None more so than the Psilocybe cubensis, the sacred shroom.
Psilocybe cubensis is a species mushroom containing the psychedelic compounds psilocybin and psilocin. Also known as "magic mushrooms" or "gold caps," psilocybe cubensis is the most well known psychedelic mushroom due to its ease of cultivation and ability to grow in many tropical geographies. Both psilocybin and psilocin are labeled as Schedule 1 drugs in the United States, but are legal in other countries (such as Brazil, Jamaica, and the Netherlands) [14]. The legal status of psilocybin-containing mushrooms is rapidly evolving, and the possession of magic mushrooms has been decriminalized in Santa Cruz and Oakland; Denver, CO; Somerville and Cambridge, Massachusetts; Washington D.C., and the entire state of Oregon). It should be noted that the cultivation, distribution, and sale of psychedelic mushrooms still remains illegal in most jurisdictions, with the exceptions of Oregon and California which legalized the use of mushrooms in clinical settings on February 1, 2021. Washington D.C. also legalized the cultivation and possession of "psychedelic and entheogenic fungi" on the same date [15]. The regulatory landscape is fragmented, confusing, and continuously evolving
Learn more about what will propel this industry into mainstream medicine
Full legalization of psychedelics for recreational use in the United States will likely follow the same trajectory as cannabis, with more liberal jurisdictions paving the way. Unlike cannabis, the medical community is pouring a tremendous amount of resources into clinical trials for multiple neurological disorders, and even physiological diseases such as cancer and stroke.
Western medicine catches on
Psychedelic-based therapies are poised to completely shift the mental health treatment paradigm. Like the cannabis industry circa mid-2000s, the first movers who assume the risk of developing these Schedule 1 pharmaceuticals will be positioned to "mop up" a highly-fragmented sector once the market catches on and there is a rush to fund the most promising medical psychedelic companies. Even though medical psychedelics are considered a novel class of drug, plant-derived psychedelic substances (e.g., psilocybin derived from "magic mushrooms") have been used for thousands of years in ancient ceremonial settings to treat both physical and mental conditions. But it wasn't until the 1940's that psychedelic research started in the West. And it wasn't even on purpose.
Read MedicalGold’s primer on the history of psychedelic use and state-of-the-science
Lysergic Acid Diethylamide (“LSD”) is probably the most widely recognized psychedelic substance (colloquially known as "acid"). While conducting research on ergot, a fungus that grows on grains and was the cause of mass poisoning in the middle ages (“Saint Anthony’s fire”), Dr. Albert Hofmann realized that the panic-inducing effects of ergot consumption could be attributed to specific alkaloids present in the plant. The basic group of the primary alkaloid (ergobasine) was discovered to be lysergic acid, and several modifications were made to produce LSD as it is known today (“LSD-25”, the 25th modification to the lysergic acid base). Dr. Hofmann produced the first chemically synthesized LSD-25 in 1938.
The exact mechanism of action was not fully understood until researchers discovered the role of serotonin in the brains of mammals ten years later (1953). LSD's chemical structure, along with the structures of many other tryptamine-based psychedelic molecules (Learn more about the science of psychedelics), looks strikingly similar to serotonin's chemical structure. The following hypothesis was put forth: LSD's mechanism of action mimics that of serotonin by binding to the same receptors and eliciting a cascade of serotonergic neuronal signaling [11]. This hypothesis was tested and it was discovered that the mind-altering effects of LSD can be attributed to an interference in serotonin signaling in the brain via competition for the 5-HT2 serotonin receptors [12]. Distributed under the name Delysid, Hofmann's LSD-25 was experimented with in clinical trials. The results were astounding. Then everything ground to a halt.
Learn more about the classification and pharmacology of psychedelics
Sixty years later and LSD has yet to reach prescription medication status. This isn't because the drug was a failure. It is because researchers simply stopped trying. A combination of new regulation (1962 Kefauver-Harris Drug Amendments requiring proof of clinical efficacy for drug approval) and loss of patent protection for Delysid destroyed the financial incentive for Sandoz labs to continue manufacturing their proprietary LSD-25. And so the research stopped. The social stigma building behind LSD didn't help either, as the Timothy Leary acolytes became the bullhorn for a countercultural revolution that glamorized "dropping out" of society. The political backlash to the movement produced the 1970 US Controlled Substances Act, which codified LSD as a "controlled substance" and enacted harsh penalties for its manufacture, distribution, and use [13].
Research on psychedelics died that day. Until now.
Data does not lie
The last decade has seen a resurgence in medical research into psychedelics and their ability to treat neurological conditions. Given its prevalence and lack of treatment options, major depressive disorder (i.e. depression) has been of particular interest. The exact molecular mechanisms that underly depression are not well understood, but researchers are quickly closing in on characterizing the differences between healthy and depressed brains.
One prevailing hypothesis is that depression is caused by "disintegration of functional neural networks," meaning regions of the brain that are supposed to activate together no longer do so. Perhaps the solution is to "jump start" the brain to reform these neural pathways?
In a recent study led by Richard Daws and Christopher Timmermann, researchers at Imperial College and Kings College (London, UK) examined the impact of orally administered psilocybin (10 and 25mg doses) on brain network organization using functional MRI (fMRI) to quantify neuronal signaling. The fMRI signals were correlated with antidepressant effects on patients as measured by Beck's depression inventory in a Phase 2 study. They discovered that the serotonin receptor 5-HT2A mediated neuronal networks became more interconnected after psilocybin dosing, indicating that psilocybin has an impact on new neural pathway formation (i.e., neuroplasticity). Importantly, they did not see these same changes in patients dosed with escitalopram, a commonly prescribed selective serotonin re-uptake inhibitor (SSRI) for depression [16]. Simply put, psilocybin is capable of rewiring your brain, and traditional antidepressants are not. This is what researchers have suspected for years; psychedelics are a "jump start" for the brain. Turns out they were right.
Learn more about the clinical trial data on medical psychedelics
Clinicians are also promoting the use of psilocybin as a means to increase the patient’s receptivity to talk therapy. A quick search of the ClinicalTrials.gov site reveals that there are 53 ongoing human clinical studies on the therapeutic effects of psilocybin-assisted therapy for a wide range of diseases, from anorexia to cancer. A Google Scholar returns over 5,000 published papers on the topic since 2018.
Here are the facts: the science is sound, the market is enormous, and the first psilocybin therapy to gain approval will be a multi-billion dollar drug.
Paving the way is Cybin, a leader in psilocybin clinical research and the sixth largest publicly traded psychedelic company (by market cap).
Cybin: On The Forefront Of Psychedelic Innovation
Drug development strategy
Cybin is on a mission to engineer novel, proprietary psychedelic therapies to revolutionize mental health treatment by considering four key principles.
They believe that a novel psychedelic-inspired compound must possess the following properties:
- Fast onset - an effective drug must "come on" quickly to reduce the lag between treatment and response. Current anti-depressants take weeks to months to start having an effect (I'm looking at you, Zoloft).
- Short duration - once the psychedelic reaches an effective concentration in the blood, it must get its job done quickly and clear from the body. Lingering drug in the blood is not a good thing, as it could distribute to the organs and have unintended side effects.
- Low variability - responses need to be predictable and consistent between doses (i.e., dose-to-dose variance) and between patients (i.e., patient-to-patient variance).
- Highly potent - "less is more"... the ideal therapeutic produces the desired response at a very low dose, reducing the potential for side effects and off target effects.
Cybin has three lead drug candidates that are being developed to treat Major Depressive Disorder (MDD), Alcohol Use Disorder (AUD), Anxiety Disorders, and Neuroinflammation.

Broadly speaking, Cybin's novel psychedelic compounds can be grouped into two major classes: indolealkylamines (tryptamines) and phenylalkylamines (phenethylamines).
CYB003 and CYB004 belong to the tryptamine group, being modified (deuterated) forms of psilocybin and dimethyltryptamine (DMT), respectively.
Related: Psychedelics: From Woodstock To Walgreens
- CYB003 is begin developed for Major Depressive Disorder (MDD) and Alcohol Use Disorder (AUD).
- CYB004 is being developed for Anxiety Disorders.
CYB005 is a phenethylamine derivative being developed for neuroinflammation.

CYB003: modified psilocybin for MDD and AUD
CYB003 is a deuterated psilocybin analog being developed for Major Depressive Disorder (MDD) and Alcohol Use Disorder (AUD). The deuteration process involves replacing hydrogen atom(s) with the deuterium isotope, which achieves more stable drug levels in the blood, shorter duration of effect, and a faster onset of action. This molecular alteration of the core psilocybin molecule is a way to shift the drug's metabolic profile while maximizing its effect on the brain. Simply put, CYB003 achieves all four tenants of Cybin's drug development strategy to develop novel psychedelic-based therapeutics with optimized pharmacokinetic properties.
Preclinical study
The company is confident that they will be able to detect positive outcomes in their Phase 1/2a study based on the success of their early preclinical investigation of CYB003, which demonstrated3:
- a well-tolerated profile following several doses in multiple species that supports repeat dosing;
- a similar in vitro and in vivo pharmacology profile when compared to psilocin, the active naturally occurring psychedelic agent in psilocybin;
- a 50% reduction in variability;
- a 50% dose reduction;
- a 50% shorter time to onset; and,
- nearly double the brain penetration indicating the potential for a less variable treatment response.

Figure 3: Variability and blood plasma concentration of CYB003 in multiple animal species. Preclinical studies of CYB003 revealed reduced variability in bioavailability between subjects and reduced levels of the drug in the blood plasma over a 4 hour time-course compared to the unmodified psilocybin molecule. This data suggests that CYB003 is absorbed into the blood stream consistently and is cleared from the bloodstream much more quickly than generic, unmodified psilocybin.

Figure 4: Improved brain to plasma ratio could result in therapeutic effects at lower doses and potential for less side effects. Preclinical studies clearly show that CYB003 crosses the blood-brain barrier much more efficiently than generic, unmodified psilocybin. This helps explain why CYB003 shows reduced levels in the blood plasma (Figure 3, above).
Phase 1/2a
Recently, the company was awarded a Schedule I license to conduct their first-in-human Phase 1/2a clinical trial1, and announced that the first two participants have been dosed in their Phase 1/2a trial evaluating CYB003 for the treatment of major depressive disorder (August 30, 2022)2. Cybin's first-in-human Phase 1/2a clinical trial is designed similarly to the psilocybin studies previously described in this article. Patients will receive two doses of either CYB003 or a placebo, then be assessed using the Montgomery-Asberg Depression Rating Scale at week 3 (following the first dose) to examine the rapid antidepressant effects of CYB003. Patients will then receive a second dose at week 6 to examine the incremental effects of an additional dose, and be assessed again at week 12 to monitor the durability of the treatment2. The detailed Phase 1/2a study protocol is available at clinicaltrials.gov under the Identifier number: NCT05385783.
“To commence dosing in our first-in-human Phase 1/2a trial is a tremendous milestone for Cybin, especially having reached the clinic within just 18 months. Our goal continues to focus on becoming a leader in creating the best psychedelic therapies for patients and today we have moved one step closer. Through our rigorous preclinical work and ongoing clinical development of CYB003, we believe we have the potential to unlock the powerful benefits of psilocybin for the treatment of MDD without its well-known limitations. The high level of participant interest in our study serves to validate the significant unmet need for alternative and better treatment options to improve mental health conditions. We expect that this Phase 1/2a trial will provide valuable insights and data. These findings will be critical in establishing a safe and efficacious treatment profile for CYB003 so we can continue to progress our mission to help revolutionize the treatment landscape for people suffering from depression."
~ Doug Drysdale, Chief Executive Officer of Cybin
CYB004: modified DMT for Anxiety Disorders
CYB004 is a deuterated form of dimethyltryptamine (DMT) being developed to treat anxiety disorders, including Generalized Anxiety Disorder GAD and Social Anxiety Disorder SAD. In its natural form, DMT is rapidly metabolized in the body and is not orally bioavailable. Cybin's preclinical studies have demonstrated that CYB004 delivered via inhalation has the potential to overcome these issues and provide increased oral and pulmonary bioavailability, faster onset with lower doses, low inter-patient variability, and better dose titration for fewer side effects and longer acting desensitization of the serotonergic receptors.
Preclinical study
The company announced the completion of their preclinical pharmacokinetic study of CYB004, delivered by inhalation, on April 13, 20223. Inhaled CYB004 demonstrated significant advantages over both IV DMT and inhaled DMT, including longer duration of action, and improved bioavailability. The study also demonstrated that inhaled CYB004 showed a similar onset of effect and dose profile to IV DMT. These data may support the potential for inhalation as a viable and well-controlled delivery system of therapeutic psychedelics.
Based on preclinical results, inhaled CYB004 demonstrated:
- Approximately 2000% improved bioavailability compared with orally administered DMT, which is known to have limited to no oral bioavailability
- Approximately 41% improved bioavailability compared with inhaled DMT
- Approximately 300% longer duration of effect when compared with IV DMT, indicating potential to extend therapeutic window
- Rapid onset of effect and similar low variability equivalent to IV DMT
“Cybin’s approach to psychedelic drug development enables the control of many biological factors, including improving the way a drug is metabolized and managing some adverse effects. In its natural form, DMT is unstable and not orally bioavailable. Based on these preclinical studies, we believe CYB004 has the potential to overcome these issues. These results also provide strong evidence that an inhaled delivery mode may be able to address the limitations of IV DMT and could be widely applicable to a variety of psychedelics."
~ Doug Drysdale, Chief Executive Officer of Cybin
Phase 1
Cybin had planned to conduct their Phase 1 clinical trial this quarter (3Q22), but instead acquired an ongoing DMT Phase 1 clinical study from Entheon Biomedical on June 7, 20224. This purchased Phase 1 study (CYB004-E) is being conducted in the Netherlands at the Centre for Human Drug Research in 50 healthy volunteers who smoke tobacco – making it the largest Phase 1 DMT clinical study conducted to date. The CYB004-E study will provide critical safety and dosing data that will replace their planned study set to begin in 3Q22. Importantly, the CYB004-E study is using unmodified, traditional DMT instead of Cybin's deuterated form (why you may ask? See below).
The study will evaluate the safety of increasing doses of DMT infusion (IV), characterize the clearance from the blood of a single dose of IV-infused DMT over 90 minutes, and determine the minimum dose needed produce a psychedelic effect. The company expects the last subject visit by November, and to complete the Phase 1 study in the first quarter of calendar 2023. Amazingly, they purchased the clinical study for only $1M with a contingent additional $0.5M for Entheon's ongoing consulting services4.
Now, you may be wondering why Cybin would purchase a Phase 1 study of an unmodified DMT drug when CYB004, their proprietary, new molecular entity is the deuterated form of DMT. The answer is two-fold. First, Cybin would have to conduct a Phase 1 study using unmodified DMT anyway in order to prove that their deuterated form, CYB004, is more effective and safer than traditional DMT. Secondly, the trial will provide dosing data that will inform the dosing of CYB004 in their secondary Phase 1 study. Basically, Cybin saved ~ seven months by purchasing an ongoing study on classic DMT that Entheon started in March, instead of having to begin their own from scratch in October of this year.
"The most precious commodity in drug development is time and acquiring this robust Phase 1 study already underway potentially accelerates the CYB004 development program by approximately nine months. The PK findings from the CYB004-E study should also help to inform the clinical path forward for this innovative and proprietary molecule. This transaction also provides Cybin with access to a world-class research foundation and the privilege to work with the Entheon team, who offer a wealth of knowledge and expertise in this psychedelic class.”
~ Doug Drysdale, Chief Executive Officer of Cybin
Pending results from the CYB004-E study, Cybin plans to evaluate CYB004 in second Phase 1 study to determine the differences between intravenous (“IV”) and inhalation routes of administration in humans. This data will be used to determine the final formulation and delivery method of CYB004, their proprietary, deuterated form of DMT.
CYB005: phenethylamine derivative for neuroinflammation
CYB005 has been flying under the radar, with basically no press other than brief mentions in CYB003 and CYB004-related news releases. Cybin's investor presentation leads us to believe that they are developing this phenethylamine derivative with a partner. It's safe to say that this drug asset is still in the preclinical stages of development. We will provide more updates as this development program gets off the ground.

Cybin's Advantage
Massive compound library
Cybin has completed more than 200 preclinical studies to date, supporting the Company's growing portfolio of proprietary psychedelic molecules. This highlights Cybin's dedication to researching and developing potential psychedelic treatments for patients in need. To date, the Company has developed over 50 novel compounds, and continues to advance preclinical work on its proprietary molecules for the treatment of neurological and mental health conditions.
Intellectual property
Cybin has 1 patent issued and 19 patents pending across 6 patent families. The Company's IP portfolio includes a United States Patent and Trademark Office patent covering certain deuterated forms of DMT and 5-MeO-DMT. This patent protects CYB004 as a putative new chemical entity. In addition, an international patent application was published by the World Intellectual Property Organization covering inhalation delivery methods for multiple psychedelic molecules.
Featuring an all-star cast
At Cybin's helm is Doug Drysdale, Chief Executive Office, who brings over 30 years' experience in the healthcare sector. Doug's experience in the industry ranges from building/turning around three pharmaceutical companies, serving as a Director on a NASDAQ-listed biotech, completing 15 corporate acquisitions, and raising $4B in both the public and private capital markets. Doug holds a bachelor’s degree in Microbial and Molecular Biology from the University of East Anglia in the U.K., and was recognized as Entrepreneur of the Year by Ernst and Young in 2012.
Second in command are Eric So, Co-Founder and President, and Aaron Baritone, Chief Operating Officer. Eric brings a wealth of capital markets experience to the team, having co-founded Trinity Venture Partners and raised hundreds of millions for companies. Like Doug, Eric has a dual academic background, holding both a BS in Anatomy and Cell Biology from McGill University and a Bachelors of Law from University of Windsor. Sitting beside Eric is Aaron Baritone, former President at UCB where he lead US commercial operations through the restructuring into CNS and Immunology Business Units (27% P&L growth, $2.2B in revenue). Like his colleagues, Aaron possesses a dynamic skillset from holding various Director-level research, regulatory, and managerial roles at Eli Lilly from 1997-2006.

Clearly, Cybin has the business development and capital markets aspects of biotech locked down tight. You couldn't ask for a more experienced and sophisticated team with well-rounded backgrounds (notice how everyone has married scientific, academic knowledge with industry and Wall Street experience).
The company's medical expertise comes from Amir Inamdar MBBS, DNB (psych), MFPM, Cybin's Chief Medical Officer. Dr. Inamdar is a psychiatrist with over 20 years of clinical and drug development experience. His expertise lies in the early stages of drug development, ranging from preclinical studies to Phase 1/2. He oversaw the development of novel therapeutics across a range of psychiatric indications including Major Depressive Disorder, narcolepsy, anxiety, schizophrenia, bipolar disorder, and substance abuse disorders. Amir's medical contributions are supported by Alex Nivorozhkin, PhD, Cybin's Chief Scientific Officer, a seasoned medicinal chemist and drug delivery expert. Dr. Nivorozhkin has lead the development of multiple new chemical entities and developed proprietary formulations for CNS drugs.

Together, these two possess the precise experience you'd want to design and lead your drug formulation and dosing studies.
Here is the full list of all 28 executives and employees: https://cybin.com/our-team/
Together, the team has more than 400 years of combined experience in drug development, with specific expertise in the science and medicine behind psychedelics and mental illness. Their track record speaks for itself, and the team boasts an impressive resume having worked at premier institutions:

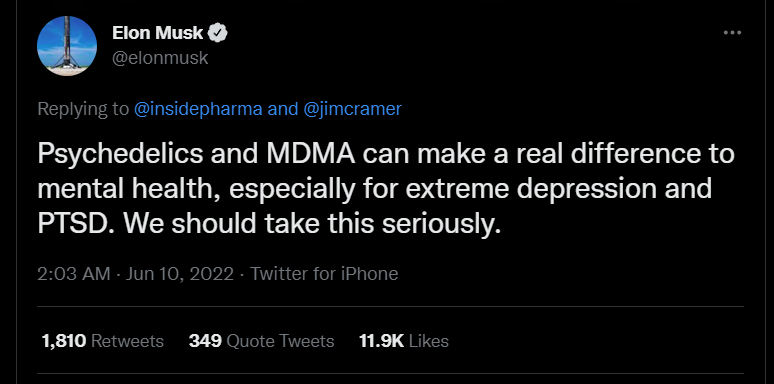
Elon's approval
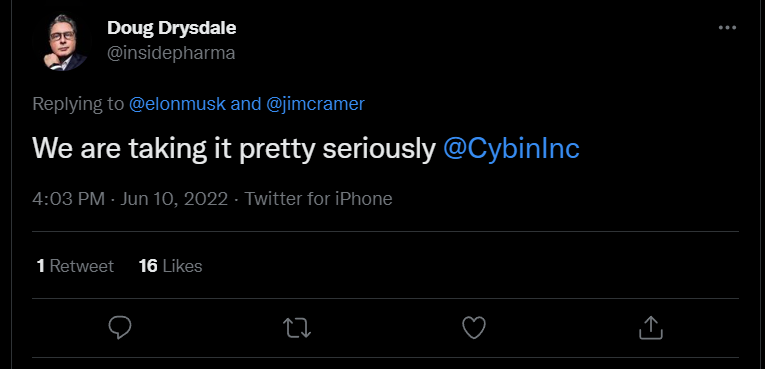
Elon Musk believes that psychedelics and other plant-based drugs are better than pharma meds in treating a number of mental health conditions5. The Tesla CEO said in a tweet last month that he had interacted with many people who have been helped by ketamine and psychedelics more than amphetamines and SSRIs. A few days ago, Musk tweeted that psychedelics and MDMA should be seriously considered for therapeutics as they can make a real difference in mental health, especially for extreme depression and PTSD. This message was a response to a tweet by Doug Drysdale, the CEO of Cybin.


Catalysts and Milestones
Recent catalysts
- Granted USPTO patent (U.S. patent 11,242,318) for deuterated DMT compound, CYB004. The allowed claims include a range of deuterated forms of DMT and 5-MeO-DMT. The patent, which is expected to expire in 2041 before consideration of any patent term extensions, covers composition of matter and protects the CYB004 drug substance as a putative new chemical entity6.
- Initiated enrollment for its first-in-human Phase 1/2a trial of CYB003 in major depressive disorder (“MDD”). The Company announced that it received a "may proceed letter" and Investigational New Drug Application clearance from the U.S. Food and Drug Administration (the "FDA") for its Phase 1/2a study in June 2022. The Phase 1/2a trial is a randomized, double-blind, placebo-controlled study evaluating up to two doses of CYB003 in approximately 32 patients with moderate to severe MDD. The Company has partnered with Clinilabs Drug Development Corporation, a full-service contract research organization with expertise in central nervous system drug development, to conduct this Phase 1/2a trial. CYB003 is designed to potentially address the challenges and limitations of oral psilocybin. Based on preclinical data, CYB003 achieved less variability in plasma levels, faster onset of action, and shorter duration of effect7.
- Initiated dosing of patients in its first-in-human Phase 1/2a trial of CYB003 in MDD. The first two human patients were dosed with CYB003, the company's deuterated psilocybin compound, in August 20222.
- Completed the acquisition of a Phase 1 DMT study from Entheon Biomedical Corp. The CYB004-E study, which is ongoing at the Centre for Human Drug Research in the Netherlands, is the largest Phase 1 DMT study conducted to date. The study is assessing the pharmacokinetics and pharmacodynamics of intravenous DMT in 50 healthy tobacco smokers, and is anticipated to provide safety and dosing data to inform the clinical development plan for CYB004. In preclinical studies, CYB004 demonstrated key advantages over intravenous and inhaled DMT, including longer duration and rapid onset of effect, improved bioavailability, and low variability.
- Reported positive preliminary data from a pilot study of Kernel Flow®, confirming Flow’s ability to successfully measure psychedelic effects on the brain. Flow is a wearable neuroimaging technology device that can quantify brain activity in real time during a psychedelic experience with the potential to support data-driven, personalized psychedelic treatments. These pilot data from an ongoing feasibility study suggested that Flow measurements confirmed changes in functional connectivity after ketamine dosing, which persisted for several days after administration. The pilot data also validated the design of the ongoing Phase 1 Flow feasibility study. They expect to report data from the Phase 1 feasibility study by the end of 2022.
- Published peer-reviewed journal article in Frontiers in Psychology introducing EMBARK, Cybin’s model of psychedelic-assisted psychotherapy. The EMBARK psychotherapy model will be used to support patients in upcoming clinical trials assessing CYB003 and CYB004 in MDD, alcohol use disorder ("AUD") and anxiety disorders. The Company has compiled a team of 28 expert faculty and advisors from leading universities and psychedelic research and training organizations for the EMBARK program9.
- Recently acquired an ongoing DMT Phase 1 clinical study from Entheon Biomedical on June 7, 20224. The data from this study will accelerate the development of Cybin's proprietary, deuterated form of DTM by approximately seven months.
Upcoming milestones
- CYB003: Deuterated psilocybin analog for the potential treatment of MDD and AUD - Cybin plans to report interim safety and PK data from the Phase 1/2a study at the end of calendar year 2022.
- CYB004: Deuterated DMT for the potential treatment of anxiety disorders. The CYB004-E Phase 1 study is currently underway, and is evaluating DMT in 50 healthy volunteers who smoke tobacco. Cybin expects the last subject visit by November, and to complete the Phase 1 study in the first quarter of calendar 2023.
- CYB005: Phenethylamine derivative for the potential treatment of neuroinflammation. Cybin expects to report preclinical data for CYB005 in the second half of calendar year 2022, at which time the Company expects to nominate a candidate and complete its assessment of the potential path forward for this candidate, including whether to develop internally or by way of a potential third party partnership.
- Kernel Flow®: Cybin and its partner Kernel expect to report data from a Phase 1 feasibility study evaluating brain activity following ketamine administration and the participant’s experience wearing the Flow headset in the fourth quarter of calendar year 2022. Results from the study will determine the next steps forward for this important program.
Financials & Stock Performance
Quarterly financials



CYBN (Daily)
CYBN completed a multi-month bottoming pattern and looks to backtest the 200-day moving average from above. The $.85 level also represents important support/resistance and we are buyers of a pullback to that support level - we take a bullish stance above $.85, however, we would become more cautious in the near term in the event that support level is breached on a weekly closing basis.

CYBN has roughly US$35 million in cash and an open US$35 at-the-market equity program that the company can use to opportunistically raise additional cash.
References
[1] Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx)
[2] America's State of Mind: Use of Mental Health Medications Increasing with Spread of Coronavirus
[4] Agarwal, S. D., & Landon, B. E. (2019). Patterns in outpatient benzodiazepine prescribing in the United States. Journal of American Medical Association, 2(1), e187399-e187399. 10.1001/jamanetworkopen.2018.7399 [Link]
[5] Alemi, F., Min, H., Yousefi, M., Becker, L. K., Hane, C. A., Nori, V. S., & Wojtusiak, J. (2021). Effectiveness of common antidepressants: A post market release study. EClinicalMedicine, 41, 101171. https://doi.org/10.1016/j.eclinm.2021.101171 [Link]
[6] Almohammed, O. A., Alsalem, A. A., Almangour, A. A., Alotaibi, L. H., Al Yami, M. S., & Lai, L. (2022). Antidepressants and health-related quality of life (hrqol) for patients with depression: Analysis of the Medical Expenditure Panel Survey from the United States. PLOS ONE, 17(4). https://doi.org/10.1371/journal.pone.0265928 [Link]
[7] Alcohol Facts and Statistics
[8] Understanding Alcohol Use Disorder
[9] Medication for the Treatment of Alcohol Use Disorder: A Brief Guide
[10] Winslow BT, Onysko M, Hebert M. Medications for Alcohol Use Disorder. Am Fam Physician. 2016 Mar 15;93(6):457-65. PMID: 26977830. [Link]
[11] Nichols DE. Psychedelics. Pharmacol Rev. 2016 Apr;68(2):264-355. doi: 10.1124/pr.115.011478. Erratum in: Pharmacol Rev. 2016 Apr;68(2):356. PMID: 26841800; PMCID: PMC4813425. [Link]
[12] Titeler M, Lyon RA, Glennon RA. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology (Berl) 1988;94:213–216. [Link]
[13] Bonson, K. R. (2017). Regulation of human research with LSD in the United States (1949-1987). Psychopharmacology, 235(2), 591–604. https://doi.org/10.1007/s00213-017-4777-4 [Link]
[14] https://en.wikipedia.org/wiki/Psilocybe_cubensis
[15] https://en.wikipedia.org/wiki/Legal_status_of_psilocybin_mushrooms
[16] Daws, R.E., Timmermann, C., Giribaldi, B. et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med28, 844–851 (2022). https://doi.org/10.1038/s41591-022-01744-z [Link]
Disclosure
Author owns shares of Cybin at the time of publishing and may choose to buy or sell at any time without notice.
DISCLAIMER:
The work included in this article is based on current events, technical charts, company news releases, and the author’s opinions. It may contain errors, and you shouldn’t make any investment decision based solely on what you read here. This publication contains forward-looking statements, including but not limited to comments regarding predictions and projections. Forward-looking statements address future events and conditions and therefore involve inherent risks and uncertainties. Actual results may differ materially from those currently anticipated in such statements. This publication is provided for informational and entertainment purposes only and is not a recommendation to buy or sell any security. Always thoroughly do your own due diligence and talk to a licensed investment adviser prior to making any investment decisions. Junior resource and biotechnology companies can easily lose 100% of their value so read company profiles on www.sedarplus.ca for important risk disclosures. It’s your money and your responsibility.
